
Spain, 17th/18th c.
polychrome, carved, turned wood; gilding
h. 142,7 cm; d. 24 cm
no. ATM 1701
Bibl.: Ratković-Bukovčan, Bogdanović, Petrinić, Desnica, Šatović, 2015., pp. 8-11; 24- 31. Lit.: Bazin, 1964, pp. 211/212; Encyclopædia Britannica: Salomónica (Accessed: March 2015); http://www.britannica.com/EBchecked/topic/519604/salomonica (Accessed: April 22nd 2015); https://www.google.hr/search?q=www.columna+salomonica (Accessed: April 2015)
Already the first steps in the conservation and restoration procedure showed that the basic construction of the column consists of two kinds of wood. Three kinds of gilding were discovered in different layers on the capital and the base, as well as the floral carvings on the column. However, the layers are not wholly preserved so that they cannot be separated. In consequence, the need arose to analyse the blue polychromy layer with marmorisation which covers the column. XRF was used to analyse the inorganic materials / pigments, and the FT-IR method was used for organic materials. It turned out that the painted layer was painted on a gypsum ground. The basic layer of light blue polychromy consists of cobalt blue and lead white, while the intensively blue marmorisation was done using smalt with cobalt oxide. All of this suggests that a massive reconstruction was done on the column, especially noticeable on the back of it, where an insertion was made of more recent wood that covers and strengthens parts of rotten wood that remained in the interior of the column.
Nada Bogdanović
senior restorer
at the Mimara Museum
The conservation/restoration procedure has included repair and chemical consolidation of the wood; darkened protective coat and the inadequate overpaint with marmorisation were removed; where possible the most recent matte gilding was removed in order for the polished gilding of better quality to be seen; visual integrity and homogenization of the form was done using a chalk preparation and repainting by water based colours. In the course of the procedure we did not decide on trying to determine what the original condition of the artefact was, because that would have doubtlessly caused decomposition and even massive damage to the artefact. Therefore we used a compromise conservation approach, which included a presentation of the information obtained through the restoration investigation and the scientific analyses.

The previous condition of the artefact – front side







Removal of the dark surface layer with the blue overpaint and white marmorisation


Scientific investigation on the twisted column from the Mimara Museum (no. ATM 1701) by using the methods of X-ray fluorescence and fourier transfer infrared spectroscopy
One of the first steps in conservation and restoration involves the appropriate and comprehensive analysis of materials. In this regard it has become very important to use modern scientific methods, instruments and new technologies; the majority of such activities are focused on elemental and chemical analysis of the paint, that is, determination of the range of dyes, pigments and adhesives used by the maker of an artefact. Investigative work on the twisted column (no. ATM 1701) involved the use of instruments for XRF analysis (X-ray fluorescence) and FT-IR (Fourier transform infrared spectroscopy), and the optical polarizing microscope. The investigations sought to determine the exact characterization of the polychromy, which is primarily blue (pigments and adhesives), and of the gilding.
Vladan Desnica, Ph.D., Associate Professor, head of the Laboratory of Conservation-Restauration Department, Academy of fine arts, Zagreb
Domagoj Šatović, Ph.D., Assistant Professor, Conservation-Restauration Department, Academy of fine arts, Zagreb
CONCLUSION
- The blue colour: XRF (X-ray fluorescence) analysis shows the use of smalt and cobalt blue, mostly mixed with lead white. A large number of admixtures in smalt is a result of the origin and production process of the pigment itself, potassium glass tinted with cobalt oxide. For instance, aluminium and iron contents depend on the origin of silicon used in the production of glass, while cobalt ore which is responsible for the colour often contains nickel, bismuth and arsenic, often in higher ratios than the cobalt itself.
- The gilding: all three samples feature pure gold on a red bolus, but different ratios of admixtures and trace elements suggest that gold leaves are of different kinds, thus confirming the assumption of the existence of several layers and interventions on the column.
- FT-IR (Fourier transform infrared spectroscopy) found the presence of two adhesives of animal origin: gelatin (Sample 2 and Sample 6) and sturgeon glue (Sample 3 and Sample 5).
- As for varnishes and resins, FT-IR analysis determined the presence of fir resins only in sample 5a. Most likely it is aged turpentine, and not real varnish.
- FT-IR analysis of pigments determined the presence of chalk and gypsum in sample 1, along with earth pigment and possibly smalt. In samples 3 and 4 basic lead sulphate-carbonate was found (Pb4SO4(CO3)2(OH)2). It is likely aged pigment of lead white. The analysis found the presence of cobalt oxide in samples 1 and 6. This is probably smalt pigment. A gypsum base was found in all the samples.
- The chemical composition of analysed materials and pigments and the chronology of their use (where relevant):
- Gypsum – CaSO4
- Cobalt blue – CoO · Al2O3; used since around 1804 to the present
- Smalt -SiO2 + K2O (10-21%) + As2O3 + CoO; in use from the 15th to the 19th century
- Lead white – PbCO3/PB(OH)2
- Bolus / red ochre – predominantly iron oxide Fe2O3
| Number | Number/name of the sample and description of the investigated area | Elements detected | Interpretation of results/comments | Macro-photograph of the sample area | Investigated sample | XRF Spectrum |
| 2 | Blue, Sample 0 | Fe, Co, Pb, Si, K, Ca, Ni, Zn, As, (S, Cu, Sr, Au, Bi) | Smalt, lead white, laid over the bolus and the gold remnants, on gypsum ground |  | 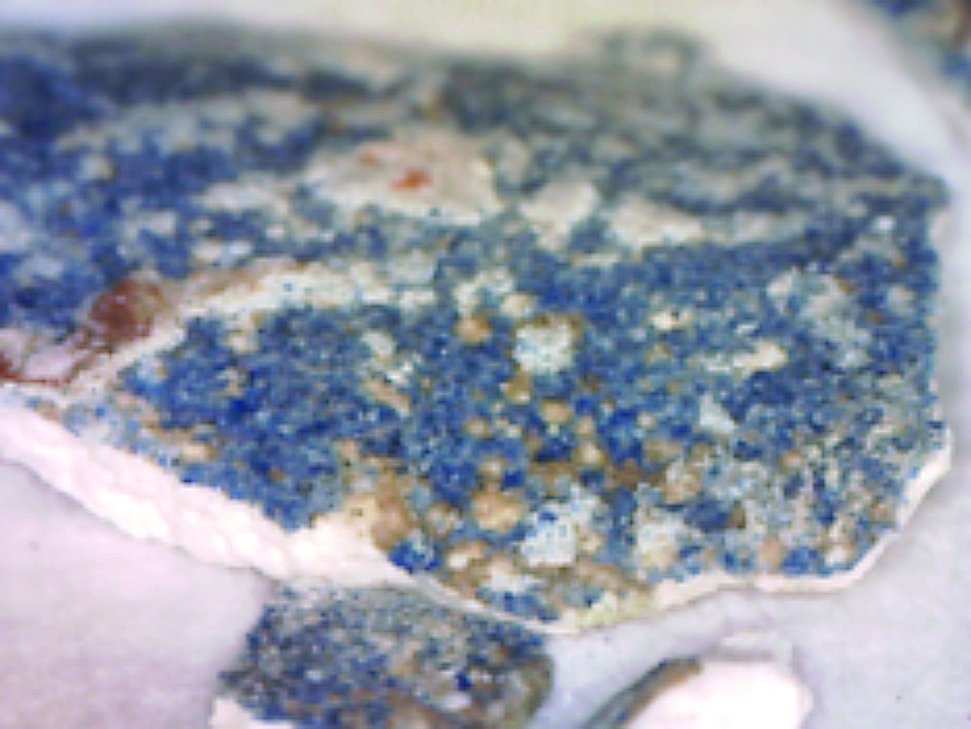 | 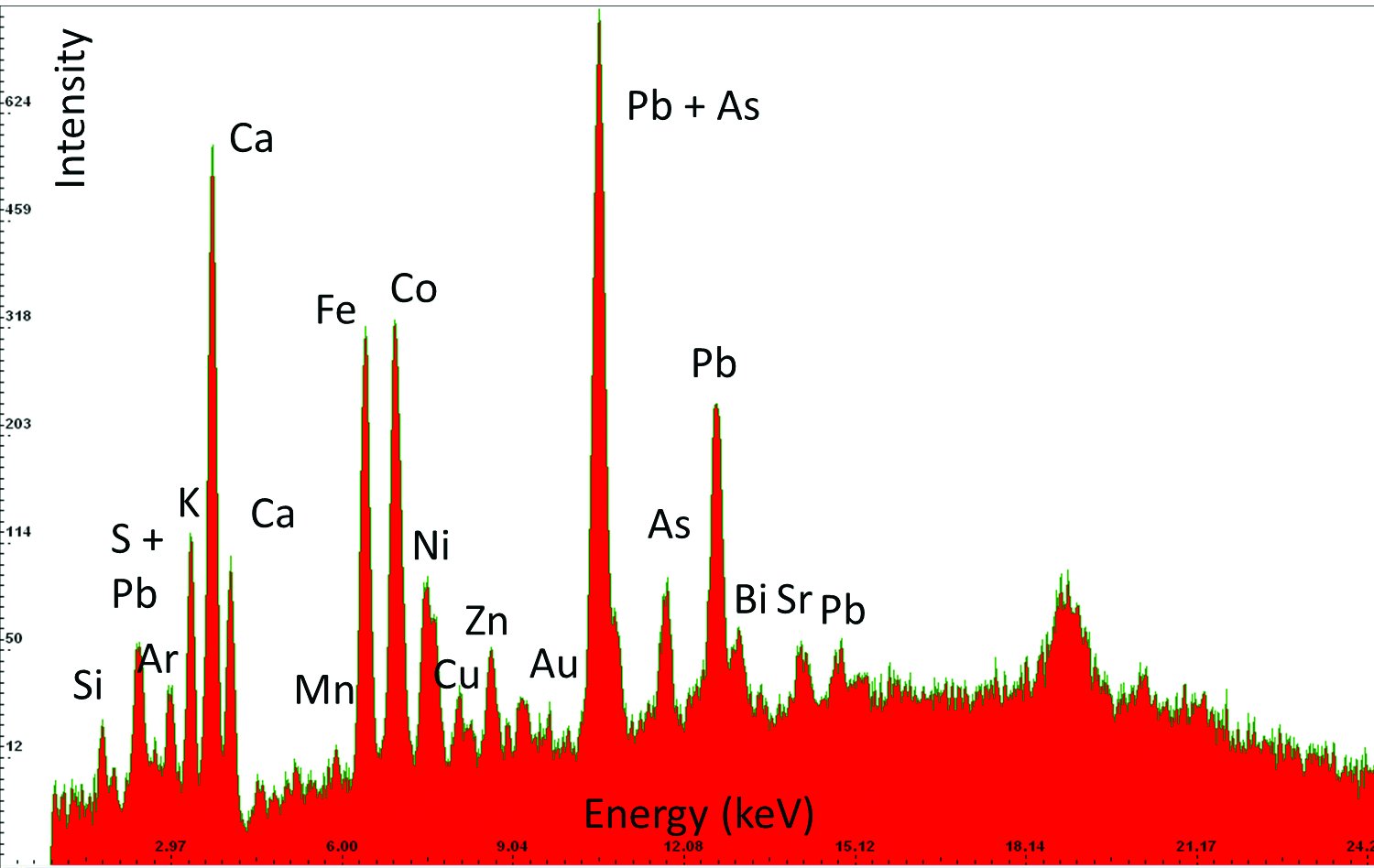 |
| 5 | Blue, Sample 3 | Pb, S, Co | Lead white, cobalt blue, on gypsum ground |  | 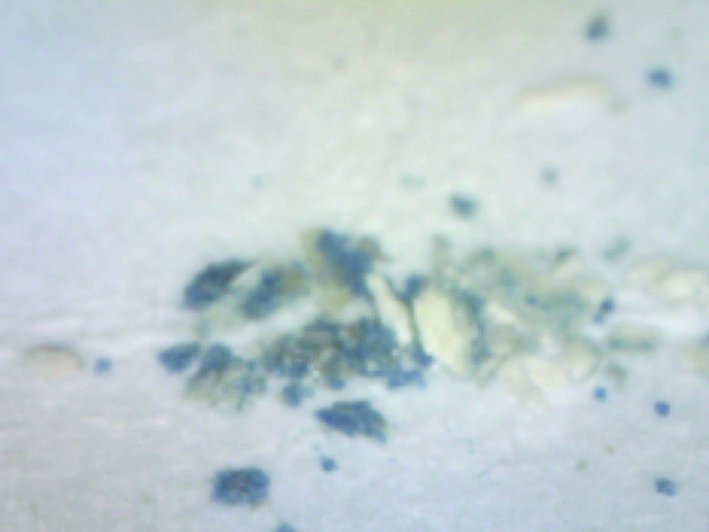 | 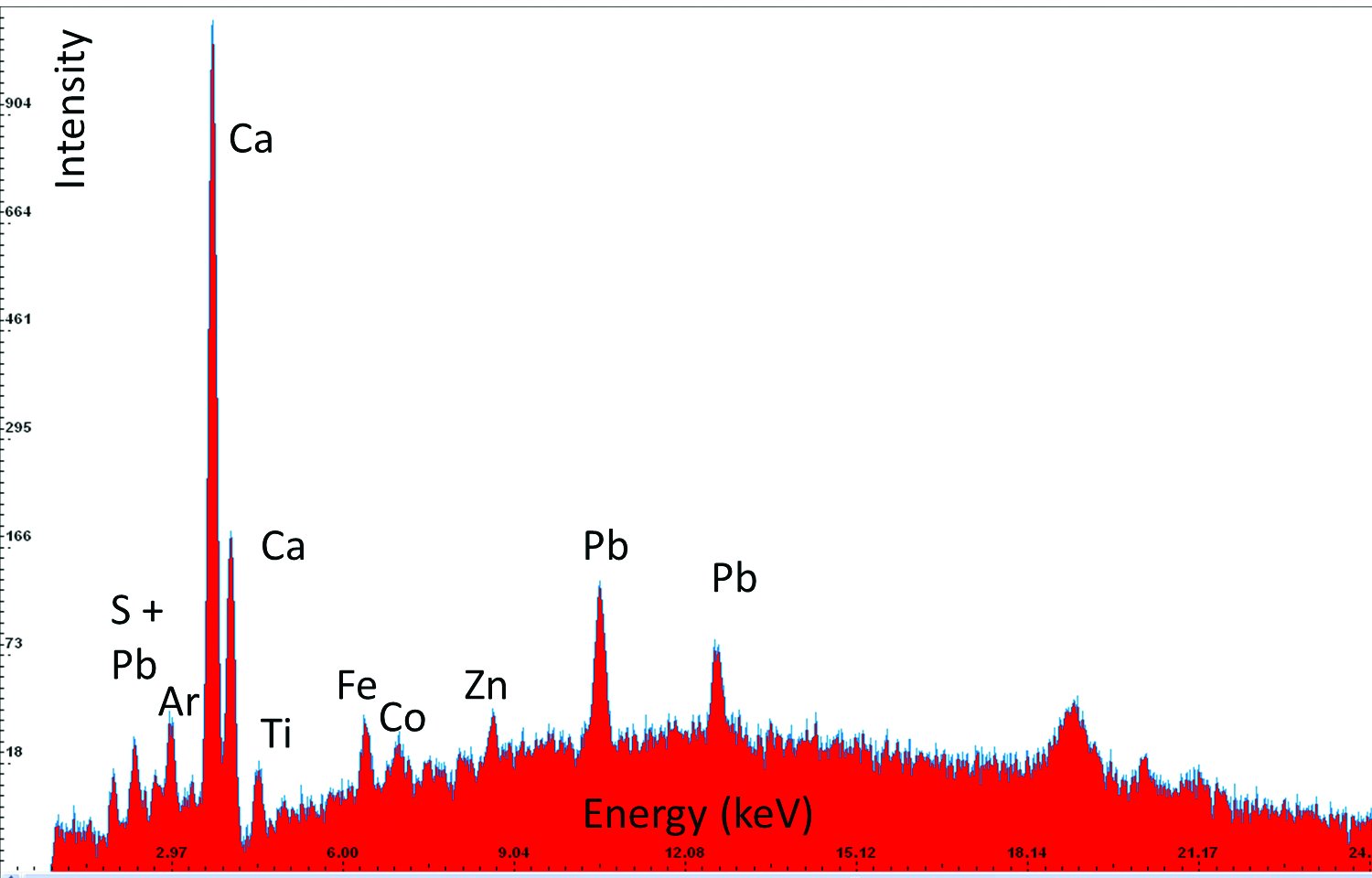 |
| 12 | Gold, Sample 9 | Au, S, Fe, (K, Ca, Ni, Cu) | Gold, on bolus and gypsum ground |  |  | 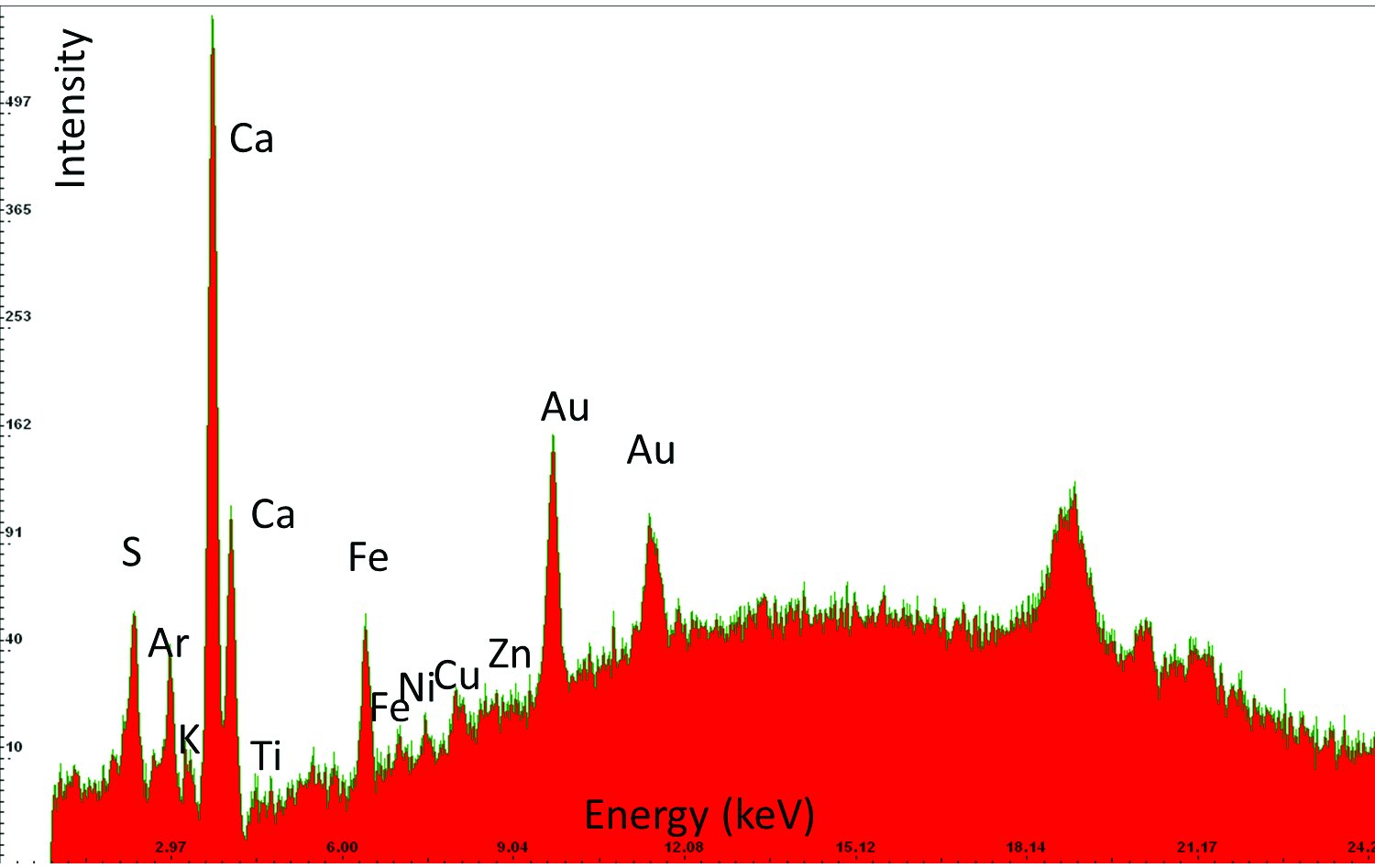 |
An example of the results obtained by X-ray fluorescence (XRF) analysis in three different areas. Based on the elements detected in the sample and their relative ratios it has been determined that the sample contains smalt, i.e, cobalt blue pigment, and gold. The column “Elements detected” lists in bold the elements detected in highest concentrations and primarily responsible for interpretation results; the other elements are listed in regular font; and the elements discovered only in traces are listed in brackets. The column “Investigated sample” shows photographs taken during the investigations, using a 92x zoom microscope integrated into the XRF device. The instrument uses a rhodium anode for primary X-ray emission, and the measurement parameters for tube voltage and filament current were 35 kV and 0.1 mA respectively. The signal collection speed was 150 cps.
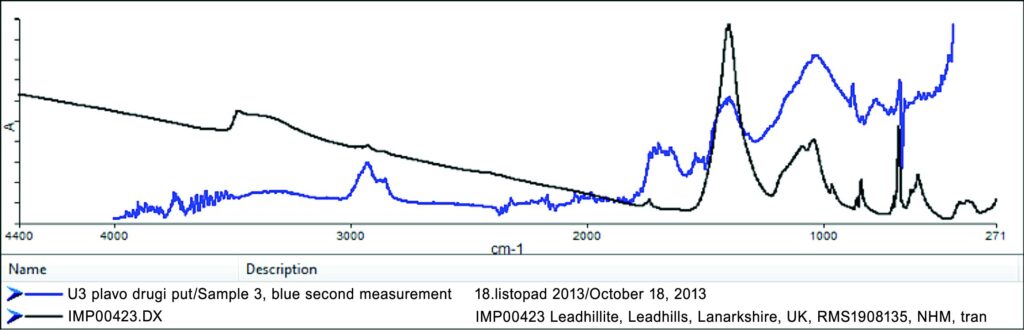
An example of the spectrum obtained by FT-IR spectroscopy on Sample 3 (blue line). A comparison with the spectra from the database (black line) determined the presence of basic lead sulphate-carbonate (Pb4SO4(CO3)2(OH)2), listed in the database as “Leadhillite.” The weak intensity of the spectrum made it impossible to precisely determine the adhesive in the sample. Part of the spectrum suggests the presence of sturgeon glue in the sample.
